4 R&D facilities with a combined 45000 Sq. ft built up area. Highly skilled and diverse team comprising of PhD’s, post graduates and chemical engineers

Expertise in handling multi-step complex chemistry, extended coupling reactions, nucleoside & carbohydrate chemistry, asymmetric catalysis, hydrogenation, organometallics and chiral products including the development of suitable analytical chiral methods

Capability to handle and develop cytotoxic intermediates/API

Capability to carry out full-scale patent evaluation including development of non-infringing processes & collaborative programs under confidentiality agreement

State-of-the art Analytical labs with NMR, ICP – OES HPLC, UPLC, GC, GC-HS, GC-MS/MS, LCMS along with stability & photo stability chambers

Capability for evaluation & development including validation of analytical test methods for Genotoxic and Nitrosamine impurities
R&D Core Competence
Carbonylations using carbon monoxide on multi kilo scale
Palladium Foray into Coupling Reactions for C-C and C-N bond formations
Capability to handle high potency molecules
LaCl3/LiCl catalysed Alkyl Grignard reactions
Cryogenic (up to-90°C), organometallic, pyrophoric reagents and reactions
- Suzuki Coupling using Miyaura borylated species
- Negishi Coupling with alkyl zinc bromides
- Stille: Between organohalides & organotin compounds
- Buchwald Hartwig: Between aryl halide & amine or aryl alcohol
- Tsuji-Trost: Between alkene and a nucleophile
- Heck- Matsuda: Between alkenes and alkyl halides
- Expertise in selection of Palladium catalyst & ligands such as
- Pd(OAc)2, Pd2(dba)3.DCM, Pd(PPh3)4, Pd(PPh3)2Cl2
- RuPhos, SPhos, X-Phos and xantphos

- Asymmetric C – C & C – N bond formation
- Asymmetric transformation
- Reductive Amination
- Chiral separations
- Gilman reaction on production scale
- Solid distillation (Upto 0.01 torr)
- Phase Transfer Catalysis
- Copper catalyzed Ullmann Reaction
Pathbreaking chemistry innovation in Camptothecin derivatives
Natural Route
Synthetic Approach
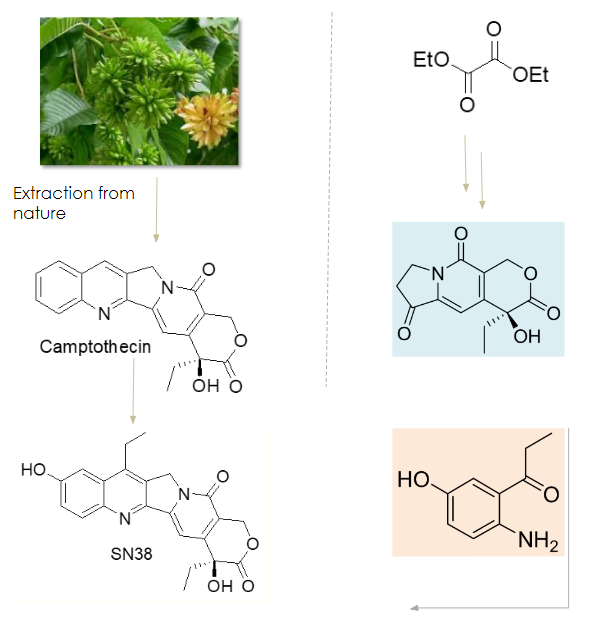
- Camptothecin is a natural alkaloid isolated from bark and stem of Camptotheca acuminate and is used for the synthesis of cytotoxic molecules like SN-38, Irinotecan etc. Irinotecan is one of the widely used chemotherapy drugs
- Use of Camptothecin from natural source has its limitation with respect to availability and quality resulting from presence of related natural products as impurities
- Cohance is the global pioneer in developing a fully synthetic route for the large scale production of Camptothecin derivative SN-38. Our proprietary technology allows us to produce SN-38 & Irinotecan with significantly higher level of purity as compared to the same from semi synthetic pathway
- We have leveraged this technology to synthesize payloads for ADCs (Antibody drug conjugates). We are supporting several innovators in their ADC clinical programs
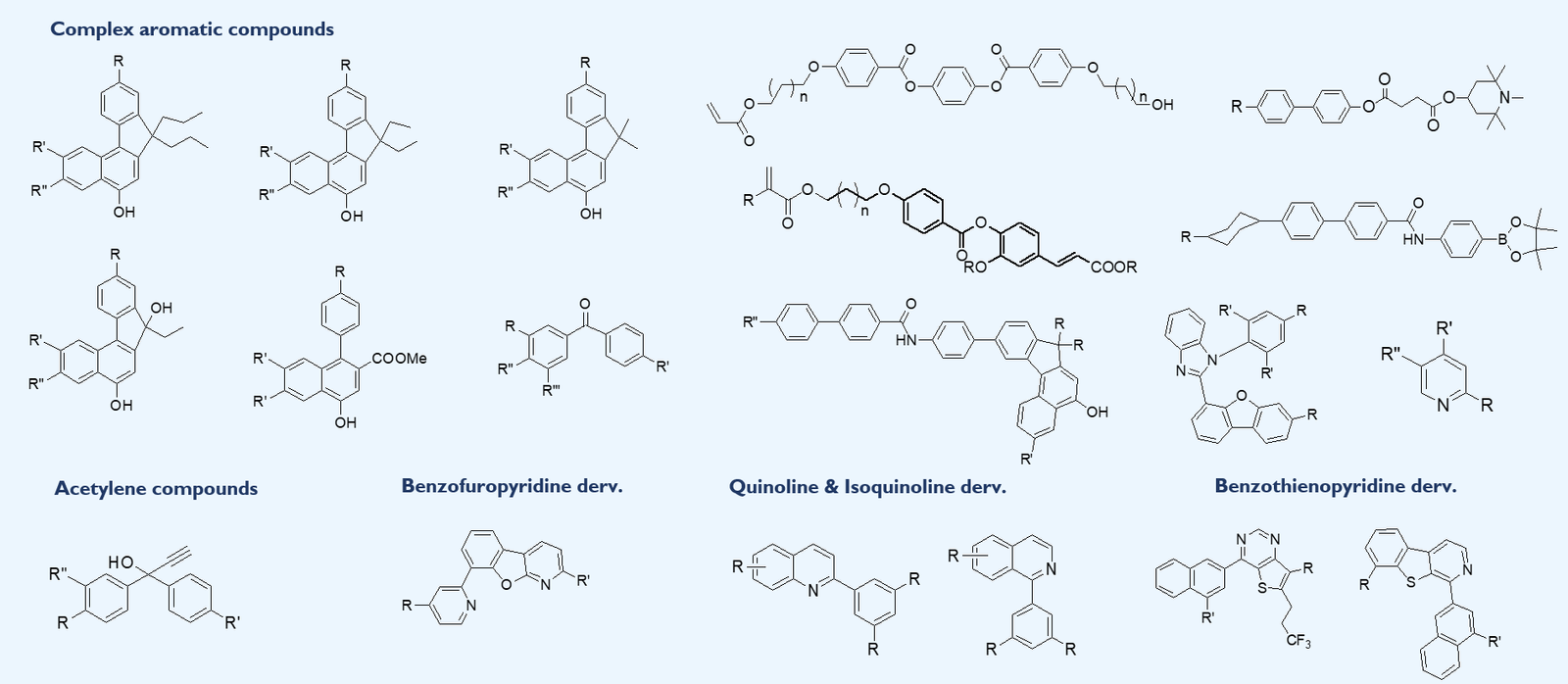
Expertise in building complex structural blocks